March 2022, v.2.5
Updated: June 2022
Feedback/Comments :: View Release Notes
- Overview
- Methodology
- Background
- Biochemical Pathway and Nutrition Treatment Rationale
- Nutrition Assessment
- Comparative Standards
- Nutrition Problem Identification
- Nutrition Intervention
- Nutrition Recommendations
- 1. Nutrition intervention for appropriate nutrient intake
- 2. Nutrition intervention to achieve appropriate blood PHE concentration
- 3. Strategies for nutrition intervention
- 4. Monitoring nutrition intervention
- 5. Nutrition intervention with alternative or adjunctive therapies
- 6. Nutrition intervention before, during and after pregnancy
- 7. Pegvaliase Therapy
- Monitoring and Evaluation
- Resources
- Benefits and Harms of Implementing the Recommendations
- Barriers to Implementation
- Areas for Future Research
- List of Tables
-
Literature Evidence Summary Tables
- T.1 Laboratory and Clinical Findings in PKU
- T.2 Classification of PKU
- T.3 Recommended Intakes of PHE, TYR and Protein for PKU Treated with Dietary Therapy
- T.4 Nutrition Problem Identification for PKU
- T.5 Comparison of Recommendations for Dietary Protein Intake for Infants and Children under 4 Years of Age to the 2015 GMDI/SERC Guideline Recommendation
- T.6 Classification of Medical Foods for PKU
- T.7 Recommendations for Nutrient Intake and Sources in the Dietary Treatment of PKU
- T.8 Monitoring Nutritional Management of PKU
- T.9 Recommendations for Neurocognitive Testing in PKU
- T.10 Recommended Dosing Regimen for Pegvaliase
PKU Toolkit Tables- T.11 Recommended Intake of PHE, TYR, Protein and Energy for an Infant with PKU
- T.12 Composition of Breast Milk, Infant Formula, and Medical Food for an Infant with PKU
- T.13 Calculation of Formula Mixture Containing Breast Milk and Medical Food for an Infant with PKU
- T.14 Calculation of Formula Mixture Containing Infant Formula and Medical Food for an Infant with PKU
- T.15 Monitoring Nutritional Management for an Infant with PKU
- T.16 Calculation of Modified Formula Mixture Using Breast Milk for an Infant with PKU
- T.17 Current Daily Nutrient Intake for an Older Infant with PKU
- T.18 Composition of Infant Formula and Medical Food for an Older Infant with PKU
- T.19 Calculation of Daily Nutrient Intake Goals for an Older Infant with PKU
- T.20 Sample Menu Introducing Solid Food for an Older Infant with PKU
- T.21 Recommended Intake of PHE, TYR, Protein and Energy for a Child, Adolescent or Adult with PKU on Dietary Therapy
- T.22 DRI Ranges for Recommended Intake of Protein and Energy for Childhood through Adulthood by Gender and Age
- T.23 Composition of Medical Food for a Child with PKU
- T.24 Calculation of Nutrient Intake Goals for a Child with PKU
- T.25 Sample Menu for a Child with PKU Eating Lunch at School
- T.26 Monitoring Nutritional Management of PKU in a Young Child
- T.27 Composition of Medical Food for a Young Adult with PKU
- T.28 Calculation of Dietary Intake for the Young Adult with PKU
- T.29 Sample Menu for a Young Adult with PKU Transitioning to Self Care
- T.30 Monitoring Nutritional Management of PKU in the Older Child, Adolescent and Adult
- T.31 Sample menu for a Young Adult with PKU Transitioning to Self Care with a Simplified Diet
- T.32 Composition of Medical Food for a Child with PKU Before Taking Sapropterin
- T.33 Calculation of Daily Nutrient Intake Goals for a Child with PKU Before Taking Sapropterin
- T.34 Baseline Blood PHE and PHE Intake for a Child with PKU Before Taking Sapropterin
- T.35 Blood PHE and Dietary PHE Intake for a Child with PKU During a Trial of Sapropterin
- T.36 Calculation of Daily Nutrient Intake Goals for a Child with PKU While Taking Sapropterin
- T.37 Sample Menu Liberalized for a child with PKU While Taking Sapropterin
- T.38 Recommended Intakes of PHE, TYR, Protein and Energy in Pregnancy for a Woman with PKU
- T.39 Composition of Medical Food in Pregnancy for a Woman with PKU
- T.40 Calculation of Daily Nutrient Intake Goals in Early Pregnancy for a Woman with PKU
- T.41 Sample Menu for a Woman with PKU in Early Pregnancy
- T.42 Recommendations for Healthy Weight Gain During Pregnancy
- T.43 Monitoring Nutritional Management of PKU During Pregnancy and Lactation
- T.44 Sample Menu for a Woman with PKU in Late Pregnancy
- T.45 Changes in Blood PHE and TYR, Dietary Intake, and Dosage for an Adult with PKU and on Dietary Therapy During a Trial of Pegvaliase
- T.46 Calculation of Dietary Intake for the Young Adult with PKU During Maintenance and Diet Adjustments on Pegvaliase
- T.47 Monitoring Nutritional Management of PKU in the Adolescent or Adult on Pegvaliase Therapy and a Normal Diet
- T.48 Recommended Intake of Protein and Energy for Adolescence through Adulthood by Gender and Age
- T.49 Changes in Blood PHE and TYR, Dietary Intake, and Dosage for an Adult with PKU on an Unrestricted Diet During a Trial of Pegvaliase
- T.50 Calculation of Daily Nutrient Intake Goals for an Adult with PKU Before Response to Pegvaliase Therapy
- T.51 Recommended Intake of PHE, TYR, Protein and Energy for an Adolescent or Adult with PKU on Dietary Therapy
- References
- Contributors
- Appendix A: Recommendation Rating Definitions
- Appendix B: Terms
- Disclaimer
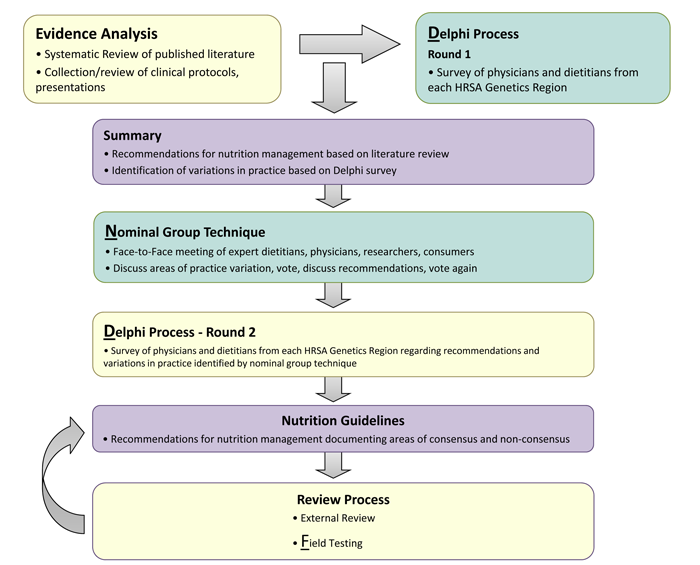
The Nutrition Guideline for PKU is an evidence- and consensus-based management guideline created through a rigorous, transparent and systematic development process. The process was adapted from the Academy of Nutrition and Dietetics with the addition of procedures to include gray literature and draw on expertise from clinical practice where research is lacking. During guideline development and updating, SERN/Emory provided infrastructure (portal development and maintenance, technology support, project coordination and management, librarian) while GMDI provided content expertise. Experienced GMDI metabolic dietitians were selected to comprise the PKU Workgroups to develop the original guideline as well as updated guidance for management of alternate pharmacotherapies (see Contributors section).The process is published in the Journal of Evaluation in Clinical Practice and is available at http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2753.2011.01807.x/pdf . The process is illustrated in Figure 1 below:
Figure 1: Nutrition Guideline Development Process
Many members of the PKU workgroup were participants in the year-long NIH sponsored project that reviewed and evaluated PKU research since 2000 and culminated with the "Phenylketonuria Scientific Review Conference: State of the Science and Future Research Needs" in February 2012. The need for evidence-based and consistent guidelines was supported by the summary findings of this project (F.2629).
PKU workgroup members independently identified questions where uncertainty or variation in practice existed. These were categorized into topics by the workgroup chairs and resubmitted to members for prioritization. From these, seven research questions were identified to guide evidence analysis and guideline development:
- For individuals with PKU, what nutrient intakes are associated with positive outcomes?
- For individuals with PKU, what blood phenylalanine concentrations are associated with positive outcomes?
- For individuals with PKU, what nutrition interventions are associated with positive outcomes?
- For individuals with PKU, monitoring of what parameters is associated with positive outcomes?
- For individuals with PKU, whose therapy includes adjunctive therapy or other therapy options (sapropterin or large neutral amino acids) what dietary considerations are needed for positive outcomes?
- For pregnant individuals with PKU, what nutritional therapies are associated with positive outcomes during pregnancy planning, pregnancy, and the post-partum period including lactation?
In 2020, workgroup members commenced an update of the PKU guidelines by adding a seventh research question:
- For individuals with PKU whose therapy includes pegvaliase as a treatment option, what medical and nutritional considerations are associated with positive outcomes?
Research questions for each topic were formulated in the PICO (population, intervention, comparison, and outcomes) format, and a separate systematic review and evidence analysis was done for each question.
The search process included both published scientific studies and gray (practice) literature.
Scientific Literature: Search terms were specific to each question, but inclusion and exclusion criteria were the same for all questions. Eligibility was limited to human studies published in English from 1980 to Fall 2000 and from 2011 to February 2014 for the original six questions, and from 2015 to 2021 for the seventh question. Additional search terms were developed specific to the addition of research question 7. There were no study design, age or setting restrictions. PubMed was the primary database used. Searches were conducted by a librarian at Emory University working in close collaboration with the project principal investigators, workgroup chairs and project coordinator. The titles and abstracts of identified articles were adjudicated by core group members for relevance and matched with inclusion/exclusion criteria. The following were considered exclusion factors:
- Animal study
- Not published in English
- Not pertaining to PKU
- Genetic studies not related to phenotype
- Case studies not specifically related to nutrition
- Not related to treatment or outcomes
- General overview intended to educate those not familiar with PKU
- Published before 2000; material assumed to have been reviewed for 2000 NIH PKU Consensus Statement.
- Reviewed for 2012 NIH State of the Science Conference and publication, or 2014 ACMG, or GMDI/SERC publications
Excluded articles were noted, with reason for exclusion documented, and qualifying articles were gathered for review and abstracting. Reference lists within the identified articles were examined for additional resources. These were added if they contributed pertinent information. Pertinent studies published before 2000 were assumed to have been reviewed for the 2000 NIH PKU Consensus Statement (L.130), and this publication is referenced in lieu of those individual articles. Studies published from 2000 through 2010 were reviewed and summarized for the NIH State of the Science Conference (Feb 2012) by dietitians who were members of the NIH Working Groups on Diet Management or Maternal PKU, and who are also involved in the GMDI/SERC effort. The summary statement from the NIH conference (F.2629) is referenced in this guideline in lieu of individual articles used in preparation of the summary statement. Similarly, the ACMG (F.2626) and GMDI/SERC (F.2627) treatment and management publications are referenced in lieu of individual articles used in their preparation. Scientific literature is referenced by "F" codes with each linked to the full article.
Gray Literature: Gray literature refers to reports of clinical practice and research that cannot be accessed through standard search systems and includes: abstracts and presentations from scientific and practice-based meetings, clinical protocols and guidelines, unpublished research, communication among experts (including listserv postings), professional newsletters, and book chapters. Work group members collected gray literature materials related to nutrition and PKU. Identified resources were screened and prioritized for inclusion based on relevance, substantive information not available in scientific literature, and currency. Gray literature is referenced by "G" codes with each linked to the full article.
Reference Materials: Reference materials refer to reports or publications not formally analyzed but used as background or supporting material within the guideline. Reference materials are referenced by the codes “L” (for peer reviewed literature) or “Y” (for non-peer reviewed literature).
Each peer-reviewed publication was critically reviewed by a trained analyst using a Quality Criteria Checklist adapted for this purpose. Quality criteria included selection and retention of subjects, appropriate controls, intervention clearly described, other intervening variables tracked, outcomes defined, measures valid and statistical analyses appropriate. Based on the number of criteria met, each article was given a quality rating of positive, neutral or negative. Workgroup members were assigned articles pertinent to a particular research question, which they reviewed and abstracted. Description included: study sample, intervention or factors of interest, findings, limitations of the study, and the article's contribution to the evidence analysis research question.
Workgroup members, using a specially designed tool, subjected eligible gray literature to similar quality assessment and abstraction.
Key information from all eligible evidence sources (peer-reviewed and gray literature) for each question was summarized. Summarized evidence was used to draft preliminary conclusion statements and recommendations.
Through two separate Delphi surveys and a nominal group process meeting clinical expertise was gathered regarding practices not addressed sufficiently in the literature, or for which evidence was inconclusive. The goal was to develop consensus or to note where consensus does not exist. Participants in the Delphi surveys were metabolic dietitians and metabolic physicians representing all geographical regions in the US. Participants in each of the nominal group meetings included: a general pediatrician, a researcher, and an advocate for the PKU community, in addition to metabolic physicians and dietitians. The Delphi and nominal group process was repeated for the addition of the seventh research question.
The final conclusion statement for each research question is based on a synthesis of evidence from peer-reviewed publications, gray literature, and Delphi and nominal group consensus techniques.
Final recommendations for the nutrition management of individuals with PKU, in each of the seven topic areas, were derived from all evidence and consensus sources. These were written, reviewed and edited by the core group. A consultant, not involved in development of the recommendations, rated each recommendation with respect to strength of the evidence (strong, fair, limited, consensus, insufficient evidence) and need for clinical action (imperative or conditional).
See Appendix A for definitions of recommendations.
These practice recommendations, along with background and other information to support their implementation are contained in the PKU Nutrition Management Guideline.
Development of guidelines is achieved with a secure, web-based application written in PHP, MySQL and hosted by Apache HTTP running on a Linux server. Guidelines for an unlimited number of IMD can be supported with the application. For each IMD, forms exist for literature management, Delphi survey and nominal group outcome management, and final guideline development. Published literature data is initially obtained via XML through the NCBI E-Utilities API. User-entered data is archived as changes are made, and content is locked through internal publishing prior to final guideline development. References for literature and tables are managed natively. Users are kept apprised of guideline development with automated email notifications. Built-in discussion forums are utilized for online group dialogue. Application security is maintained through use of SSL, user accounts and user-level access controls for each form. Backups are performed nightly. The complete PKU Nutrition Management Guideline can be accessed through both the SERC and GMDI websites.
As a result of the evidence analysis and consensus processes, important areas where research is needed were identified. Further, the practice recommendations outlined should lead to greater standardization of care and enable outcomes studies within and across centers. This guideline will be reviewed every 2 years and updated when warranted by new developments in PKU research or change in accepted clinical practice. The guideline will be updated following the processes of publication search then formal evidence and consensus analysis used for original guideline development. The addition of a seventh research question regarding alternate therapy constitutes the first update to this guideline.


