February 2019, v.1.0
Current version: v.1.4
Updated: February 2019
Feedback/Comments :: View Release Notes
- Overview
- Methodology
- Background
- Biochemical Pathway and Nutrition Treatment Rationale
- Nutrition Assessment
- Comparative Standards
- Nutrition Problem Identification
- Nutrition Intervention
- Nutrition Recommendations
- Monitoring and Evaluation
- Resources
- Benefits and Harms of Implementing the Recommendations
- Barriers to Implementation
- Areas for Future Research
- List of Tables
-
Literature Evidence Summary Tables
- T.1 VLCAD Phenotypes
- T.2 Laboratory and Clinical Findings in Severe VLCAD Deficiency
- T.3 Recommended Intakes of Essential Fatty Acids for Individuals with VLCAD
- T.4 Nutrition Problem Identification for VLCAD
- T.5 Medical Foods for the Nutrition Management of VLCAD
- T.6 Sources of Medium Chain Triglycerides (MCT)
- T.7 Recommendations for Fasting Intervals for Individuals with VLCAD when well
- T.8 Recommended Fat (total, long chain and medium chain), Energy and Protein Intakes for Individuals with VLCAD when Well
- T.9 Essential Fatty Acid Content of Selected Oils
- T.10 Monitoring the Nutritional Management of an Individual with VLCAD when Well
- T.11 Long-chain Fat Restriction and MCT Supplementation Recommendations for Individuals with VLCAD over the age of 1 year
- T.12 Long-chain Fat Restriction and MCT Supplementation Recommendations for Infants with VLCAD
- T.13 MCT Use in Individuals with VLCAD and Cardiomyopathy and/or Rhabdomyolysis
- T.14 European Consensus of Essential Fatty Acid Intakes
- References
- Contributors
- Appendix A: Recommendation Rating Definitions
- Appendix B: Terms
- Disclaimer
The Nutrition Management Guideline for VLCAD is an evidence- and consensus-based management guideline created through a rigorous, transparent and systematic development process. The process was adapted from the Academy of Nutrition and Dietetics (L.348); but incorporates the use of grey literature (described below) and the addition of explicit consensus techniques to draw on the experience and expertise from clinical practice to fill in gaps where research is lacking. The process has been published in the Journal of Evaluation in Clinical Practice (L.347) and is available at http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2753.2011.01807.x/pdf. The process is illustrated in Figure 1,
Figure 1: Nutrition Guideline Development Process
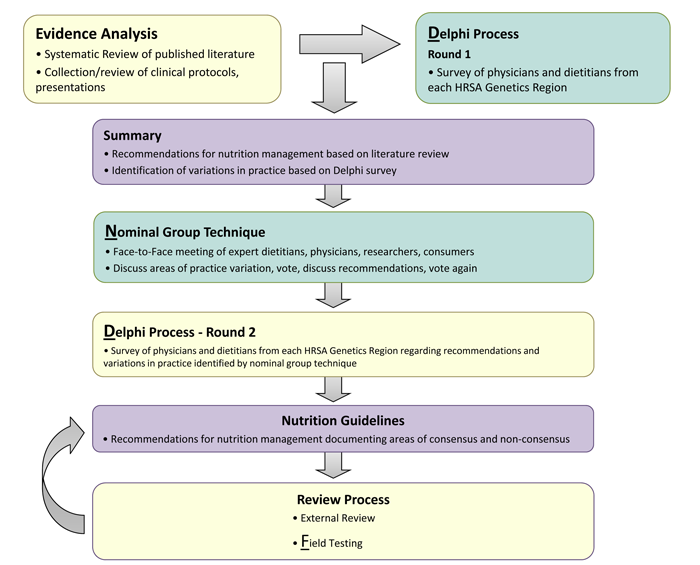
VLCAD work group members (listed in Contributors section) independently identified questions where uncertainty and/or variation in practice existed. These were categorized into topics by the work group chair and resubmitted to members for prioritization. Six topics were identified for evidence analysis and guideline development:
- Nutrient intake and nutrition management approaches when well
- Nutrition management during illness
- Nutrient supplementation and other treatments
- Monitoring
- Exercise
- Menstruation, pregnancy, delivery and the post-partum period
Research questions for each topic were formulated in the PICO (population, intervention, comparison, and outcomes) format. Separate systematic review and evidence analysis were completed for each question.
The six research questions were:
- For healthy individuals with VLCAD, what nutrient intake goals and nutrition management approaches when well are associated with positive outcomes?
- Forindividuals with VLCAD, what nutrition interventions are associated with positive outcomes during illness (including cardiomyopathy and rhabdomyolysis), surgery or other stress?
- For individuals with VLCAD, do other specific nutrient supplementation or other treatment modalities (i.e. L-carnitine, triheptanoin, bezafibrates) improve outcomes?
- For individuals with VLCAD, monitoring of which parameters is associated with positive outcomes?
- Forindividuals with VLCAD, what nutrition interventions are associated with positive outcomes during exercise?
- Forwomen with VLCAD, what nutrition interventions are associated with positive outcomes during menstruation, pregnancy, delivery and the post-partum period?
Positive outcomes include improvements in or maintenance of normal metabolic, clinical, and nutritional status (including growth and nutrient intake).
Because of the scarcity of published scientific literature in nutrition management of IMDs, the search process included both published scientific studies and gray (practice) literature.
Scientific Literature: Search terms were specific to each question, but inclusion and exclusion criteria were the same for all questions. Eligibility was limited to human studies published in English from 1990 to Fall 2016. There were no study design, age or setting restrictions. PubMed was the primary database used. Searches were conducted by a librarian at Emory University working in close collaboration with the project principal investigators, workgroup chairs and project coordinator. The titles and abstracts of identified articles were adjudicated by workgroup co-chaiirs for relevance and matched with inclusion/exclusion criteria. The following were considered exclusion factors:
-
Animal study
-
Not published in English
-
Not pertaining to VLCAD
-
Genetic studies not related to phenotype
-
Case studies not specifically related to nutrition
-
Not related to treatment or outcomes
-
General overview intended to educate those not familiar with VLCAD
Excluded articles were noted, with reason for exclusion documented, and qualifying articles were gathered for review and abstracting. Reference lists within the identified articles were examined for additional resources. These were added if they contributed pertinent information. Scientific literature is referenced by "F" codes with each linked to the full article.
Gray Literature: Gray literature refers to reports of clinical practice and research that cannot be accessed through standard search systems and includes: abstracts and presentations from scientific and practice-based meetings, clinical protocols and guidelines, unpublished research, communication among experts (including listserv postings), professional newsletters, and book chapters. Work group members collected gray literature materials related to nutrition and VLCAD. Identified resources were screened and prioritized for inclusion based on relevance, substantive information not available in scientific literature, and currency. Gray literature is referenced by "G" codes with each linked to the full document.
Reference Materials: Reference materials refer to reports or publications not formally analyzed but used as background or supporting material within the guideline. Reference materials are referenced by the codes "L" (for peer reviewed literature) or "Y" (for non-peer reviewed literature).
Search Results: The search for scientific literature identified 888 titles. After duplicate entries and unavailable sources removed, the titles and abstracts of 687 articles were reviewed for relevance to the nutrition management guideline questions. At this step 593 articles were excluded and the remaining 94 were assigned to questions, appraised for quality, and abstracted. Reasons for exclusion were coded and included: 125-published before 1990, 27-nonEnglish, 18-genetic studies, 7-general overview article intended for education, 407 animal or invitro studies not related to VLCAD treatment or outcomes, and 400 unrelated to VLCAD treatment or outcomes.
The search for gray literature identified 26 sources. These were appraised and abstracted. Later three were excluded due to lack of relevance to the nutrition guideline research questions.
Each included formal and gray literature source (total 117) was assigned to one or more research questions with the following result:
- 57 Nutrient intake and nutrition management approaches when well
- 90 Nutrition management during Illness
- 57 Nutrient supplementation and other treatments
- 54 Monitoring
- 52 Exercise
- 6 Menstruation, pregnancy, delivery and the post-partum period
Each peer-reviewed publication was critically reviewed by a trained analyst using a Quality Criteria Checklist adapted for this purpose. Training and role of analysts was published in 2015 (L.349). Quality criteria included selection and retention of subjects, appropriate controls, intervention clearly described, other intervening variables tracked, outcomes defined, use of valid measures valid and appropriate statistical analyses. Based on the number of criteria met, each article was given a quality rating of positive, neutral or negative.
Of 94 articles appraised, 24% were rated positive, 65% rated neutral and 11% rated negative quality.
For the evidence abstraction step, workgroup members were assigned articles pertinent to a particular research question, which they reviewed and abstracted to an evidence table. Abstraction included: study sample, intervention or factors of interest, findings, limitations of the study, and the article's contribution to the evidence analysis research question.
Workgroup members, using a specially designed grey literature quality checklist tool, subjected eligible gray literature to similar quality assessment and abstraction.
The sources of scientific evidence available consisted of 4 randomized control trials, 6 non-randomized or non-controlled trials, 2 cohort studies, 2 case control studies, 1 study of diagnostic testing, 10 cross-sectional studies or surveys, 60 case reports (64%), and 4 reviews and 2 letters to editor.
Key information from all eligible evidence sources (scientific and gray literature) for each question was summarized on an overview/evidence table. Evidence was then synthesized into evidence summaries by topic and a preliminary conclusion statement for each question was drafted by a work group member. During this time the first round of a Delphi survey was conducted to gather input for areas of uncertainty and variation in practice from experts in VLCAD, including metabolic dietitians and metabolic physicians, from all geographic regions of the United States. Survey items were structured to elicit comment on and level of agreement/disagreement (7-point scale) with practice approaches. Consensus was defined as 80% agreement. Delphi 1 findings were added as another form of evidence.
The synthesis of evidence and preliminary conclusion statement for each question and draft recommendations were discussed by the entire workgroup and finalized by the work group chairs and the Core Group (consiting of principle investigators, workgroup chairs and a consultant). These documents were provided, two weeks in advance, to experts (which included researchers, parent advocate for the VLCAD community, metabolic physicians and metabolic dietitians) invited to participate in the nominal group process meeting.
At a two-day nominal group meeting, key issues and controversies associated with each of the research questions were presented along with available evidence and preliminary recommendations. These issues were deliberated among participants using the facilitation methods of nominal group process. Voting and further discussion were used to resolve differences and determine level of consensus. Proceedings of the nominal group meeting were recorded for future reference.
The Delphi 2 survey was prepared and distributed to a separate set of experts to gather further input and determine level of agreement/disagreement regarding issues for which evidence was inconclusive and/or consensus lacking.
Through two separate Delphi surveys and a nominal group process meeting, clinical expertise was gathered regarding consensus-based practices not addressed sufficiently in the literature, or for which evidence was inconclusive. The goal was to develop consensus or to note where consensus does not exist.
The final conclusion statement for each research question is based on a synthesis of evidence from peer-reviewed publications, gray literature, and Delphi and nominal group consensus techniques.
Final recommendations for the nutrition management of individuals with VLCAD, in each of the six topic areas, were derived from all evidence and consensus sources. These were written, reviewed and edited by the core group. The co-investigators and a consultant independently rated each recommendation with respect to strength of the evidence for the recommendation (strong, fair, limited, consensus, insufficient evidence) and need for clinical action (imperative or conditional) using criteria developed by the American Academy of Pediatrics (L.350). Any rating discrepancies were discussed and resolved.
See Appendix A for recommendation rating definitions.
For each question, a conclusion statement, specific practice recommendations, and evidence is detailed in the Nutrition Recommendations section of this guideline. Background and other information to support implementation of the recommendations are presented in other sections of this VLCAD Nutrition Management Guideline.
Development of the nutrition managment guidelines is achieved with a secure, web-based application written in PHP, MySQL and hosted by Apache HTTP running on a Linux server. Guidelines for an unlimited number of IMD can be supported with the application. For each IMD, forms exist for literature management, Delphi survey and nominal group outcome management, and final guideline development. Published literature data is initially obtained via XML through the NCBI E-Utilities API. User-entered data is archived as changes are made, and content is locked through internal publishing prior to final guideline development. References for literature and tables are managed natively. Users are kept apprised of guideline development with automated email notifications. Built-in discussion forums are utilized for online group dialogue. Application security is maintained through use of SSL, user accounts and user-level access controls for each form. Backups are performed nightly.
The complete VLCAD Nutrition Management Guideline can be accessed through both the SERC and GMDI websites.
As a result of the evidence analysis and consensus processes, important areas where research is needed were identified. Further, the practice recommendations outlined should lead to greater standardization of care and enable outcomes studies within and across centers. This guideline will be reviewed every 2 years, and updated when warranted by new developments in VLCAD research or change in accepted clinical practice. The guideline will be updated following the processes of publication search, then formal evidence and consensus analysis used for original guideline development.


