March 2017, v.1.2
Updated: September 2017
Feedback/Comments :: View Release Notes
- Overview
- Methodology
- Background
- Biochemical Pathway and Nutrition Treatment Rationale
- Nutrition Assessment
- Comparative Standards
- Nutrition Problem Identification
- Nutrition Intervention
- Nutrition Recommendations
- Monitoring and Evaluation
- Resources
- Benefits and Harms of Implementing the Recommendations
- Barriers to Implementation
- Areas for Future Research
- List of Tables
-
Literature Evidence Summary Tables
- T.1 Clinical Symptoms and Laboratory Findings in PROP
- T.2 Nutrition Problem Identification for PROP based on the Academy of Nutrition and Dietetics Nutrition Care Process
- T.3 Recommended Intakes of PRO and Energy for Well Individuals with PROP
- T.4 Classification of Medical Foods for the Nutrition Management of PROP
- T.5 Nutrient Sources in the Nutrition Management of Well Individuals with PROP
- T.6 Nutrition Management of Individuals with PROP
- T.7 Monitoring the Nutritional Management of Well/Stable Individuals with PROP
- References
- Contributors
- Appendix A: Recommendation Rating Definitions
- Appendix B: Terms
- Disclaimer
The Nutrition Guideline for PROP is an evidence- and consensus-based management guideline created through a rigorous, transparent and systematic development process. The process was adapted from the Academy of Nutrition and Dietetics with the addition of specific techniques to draw on the expertise from clinical practice to fill in gaps where research is lacking. The process has been published in the Journal of Evaluation in Clinical Practice and is available at http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2753.2011.01807.x/pdf. The process is illustrated in Figure 1, below:
Figure 1: Nutrition Guideline Development Process
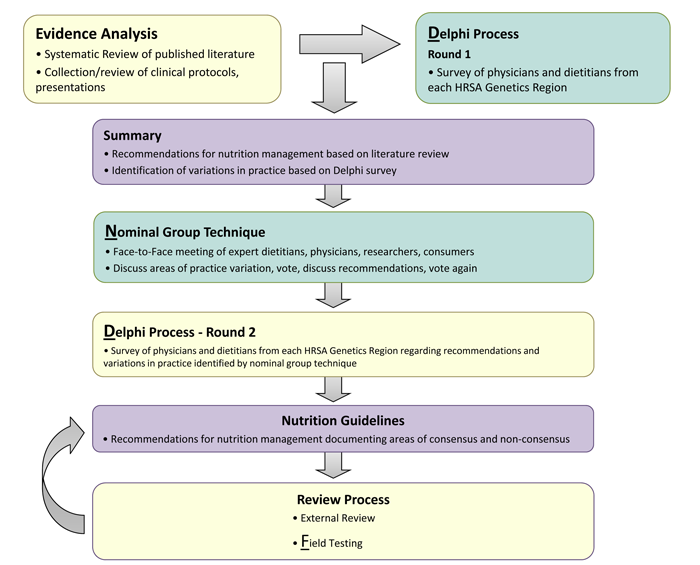
Six PROP work group members independently identified questions where uncertainty and/or variation in practice existed. These were categorized into topics by the work group chair and resubmitted to members for prioritization. Seven topics were identified for evidence analysis and guideline development:
- Recommended nutrient intake
- Nutrition interventions
- Nutrient supplementation and other treatments
- Monitoring
- Menstruation, pregnancy, the postpartum period, and lactation
- Secondary complications
- Liver transplantation
Research questions for each topic were formulated in the PICO (population, intervention, comparison, and outcomes) format. Separate systematic review and evidence analysis were done for each question.
The seven research questions were:
1. For individuals with PROP, what nutrient intakes are associated with a positive outcome?
2. For individuals with PROP, what nutrition interventions are associated with a positive outcome?
3. For individuals with PROP, do specific nutrient supplementation or other treatment modalities improve outcome?
4. For individuals with PROP, monitoring of which parameters is associated with a positive outcome?
5. For women with PROP, what nutrition therapies during menstruation, pregnancy, the post-partum period and lactation are associated with a positive outcome?
6. For individuals with PROP with secondary complications, what nutrition intervention are associated with a positive outcome?
7. For individuals with PROP undergoing liver transplantation, what nutrition interventions pre-, during and post-transplant result in a positive outcome?
Because of the known scarcity of published scientific literature in nutrition management of IMDs, the search process included both published scientific studies and gray (practice) literature.
Scientific Literature: Search terms were specific to each question, but inclusion and exclusion criteria were the same for all questions. Eligibility was limited to human studies published in English from 1985 to summer 2015, with nutrition data included. There were no study design, age or setting restrictions. PubMed was the primary database used. Searches were conducted by a librarian at Emory University working in close collaboration with the project principle investigators, work group chairs and project coordinator. The titles and abstracts of identified articles were scanned for relevance and matched with inclusion/exclusion criteria by the work group member responsible for the question. Excluded articles were noted and qualifying articles were gathered for review and abstracting.
Gray Literature: Gray literature refers to reports of clinical practice and research that cannot be accessed through standard search systems and includes abstracts and presentations from scientific and practice-based meetings, clinical protocols and guidelines, unpublished research, communication among experts (including listserves), professional newsletters, and book chapters. The search for gray literature involved request to individuals (e.g., practitioners and researchers) and organizations for materials related to nutrition and PROP. Identified resources were screened and prioritized for inclusion based on relevance, substantive information not available in scientific literature, and currency.
Each scientific article was critically reviewed by a trained analyst using a Quality Criteria Checklist. Quality criteria addressed: selection and retention of subjects, groups comparable, intervention clearly described and followed, other intervening variables tracked, outcomes defined, measures valid, and appropriate statistical analysis. Based on number of criteria met, each article was assigned a quality rating of positive, neutral or negative. Workgroup members then abstracted sample size and characteristics, intervention and outcome findings, and author’s conclusions to Overview Tables.
Gray/practice resources were reviewed by work group members using a specially developed quality criteria checklist for gray literature that included: clear purpose, relevance to intended users, systematic development process, clear clinical recommendations, applicable to practice, and free of conflict of interest. They abstracted clinical practices and outcome information onto Overview Tables.
Key information from all eligible evidence sources (scientific and gray literature) for each question was summarized on an overview/evidence table. Evidence was then synthesized into evidence summaries by topic and a preliminary conclusion statement was drafted by a work group member. These were discussed by the entire workgroup and finalized by the work group chairs and the Core Team.
Consensus-based input: Many issues of concern to nutrition management were not addressed or were inconclusive from scientific and gray literature. For these issues, expert input from nutrition and medical clinicians and researchers was sought using an initial Delphi survey, nominal group process meeting, and a second-round Delphi survey. By systematically employing these techniques, the level of agreement with specific practice statement was quantified.
Input of key stakeholders (patients and their families): Two parents and members of the Organic Acidemia Association and the Propionic Acidemia Foundation respectively, participated in the nominal group process meeting.
The final conclusion statement and evidence summary for each question represents a synthesis of evidence from scientific publications, gray literature, and delphi and nominal group consensus techniques.
Specific recommendations for nutrition management in each of the seven topic areas were derived by the PROP work group, in consultation with the Core Team, utilizing the evidence summaries, conclusion statements and the results of the consensus-based input. Each recommendation was rated with respect to strength of the body of evidence (strong, fair, weak, consensus, insufficient evidence) and need for clinical action (imperative or conditional). See Appendix A: Recommendation Rating Definitions.
These practice recommendations along with background and other information to support their implementation are contained throughout the Guideline for Nutrition Management of Individuals with PROP document. The final document was reviewed, using AGREE II criteria, by an external panel of metabolic dietitians, physicians and an expert in guideline development methodology who were not involved in the evidence analysis or guidelines development phases. When finalized, the guideline will be available for dissemination and field-testing. All Guidelines for Nutrition Management of Individuals with Inherited Metabolic Disorders can be accessed through the Southeast Regional Newborn Screening and Genetics Collaborative (SERC) and Genetic Metabolic Dietitians International (GMDI) websites.
Production of guidelines is achieved with a secure, web-based application written in PHP, MySQL and hosted by Apache HTTP running on a Linux server. Guidelines for an unlimited number of IMD can be supported with the application. For each IMD, forms exist for literature management, Delphi and Nominal survey management, and final guideline development. Published literature data is initially obtained via XML through the NCBI E-Utilities API. User-entered data is archived as changes are made, and content is locked through internal publishing prior to final guideline development. References for literature and tables are managed natively. Users are kept apprised of guideline development with automated email notifications. Built-in discussion forums are utilized for online group dialogue. Application security is maintained through use of SSL, user accounts and user-level access controls for each form. Backup is performed nightly. The complete PROP Nutrition Management Guideline can be accessed through both the SERC and GMDI websites.
As a result of the evidence analysis and consensus processes, important areas for future research were identified. Further, the practice recommendations outlined should lead to greater standardization of care and enable outcomes studies within and across centers. When warranted by developments in PROP research and clinical practice, this guideline will be updated.
An oversight committee reviews the status of Nutrition Management Guidelines for Individuals with IMD on an annual basis. A search for new literature is conducted, and a determination made in conjunction with the workgroup chair regarding the need for a topic or full guideline update. All guidelines are updated at least every five years (after initial release) following the methodology described above.


